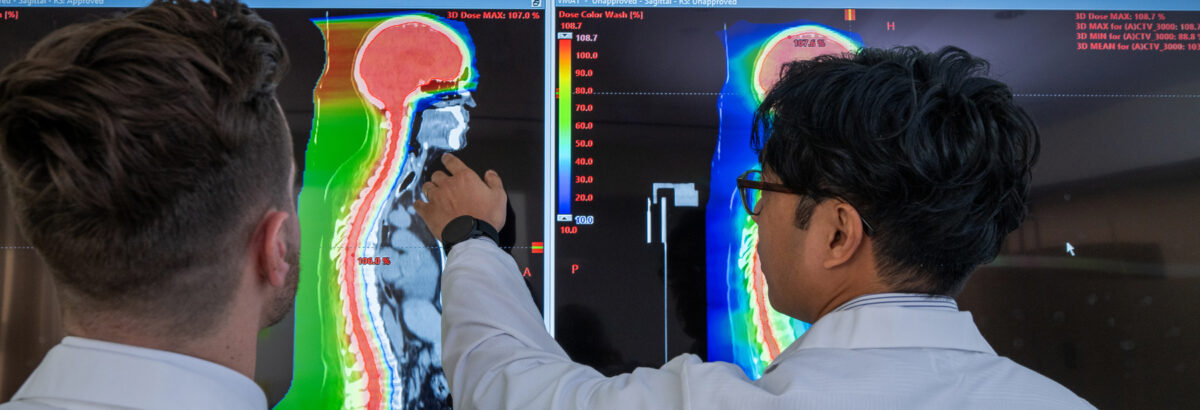
Lung and Thoracic Tumors FAQs
Frequently Asked Questions
These answers to frequently asked questions can help you decide whether proton therapy for lung and thoracic tumors is right for you.
What types of lung and chest tumors can be treated with proton therapy?
Proton therapy is a noninvasive treatment option for chest tumors of all sizes including:
– Non-small lung cancer
– Small cell lung cancer
– Malignant pleural mesothelioma
– Thymomas and thymic carcinomas
– Cardiac tumors
– Thoracic sarcomas
– Recurrent thoracic cancers
What are the benefits of proton therapy for thoracic cancers?
When lung cancer is treated with conventional radiation, it is difficult to deliver a high enough radiation dose to control the cancer without also damaging the normal lungs, esophagus, heart and spinal cord. Because proton therapy is extremely precise, radiation oncologists can treat the tumor with a higher dose of radiation and significantly reduce the amount of radiation delivered to healthy organs and tissues. Proton therapy for thoracic malignancies optimizes the chances of a cure but with less risk of side effects such as lung inflammation (pneumonitis) or scarring (fibrosis), difficulty swallowing, heart complications such as pericarditis, and other complications that are commonly seen with conventional radiation therapy.
Are there side effects?
Side effects of proton therapy depend on the exact area of the body receiving treatment, the tumor size, and the types of healthy tissues near the tumor. The most common side effect of radiation therapy for lung and thoracic tumors includes fatigue, skin irritation, and temporary change in swallowing or cough. Your doctor will help manage any side effects during and after proton therapy.
Can I have proton therapy if I had previous radiation to the chest for another type of cancer?
Yes—patients who previously received radiation for breast, lung or esophageal cancer, or for lymphoma, are often particularly good candidates for proton therapy to treat a new or recurrent lung or thoracic tumor. This is because of proton therapy’s precision and ability to target the tumor with significantly less radiation to nearby tissues and organs, allowing for more radiation dose to be focused to the tumor and a better chance of a cure.
How many treatments are required?
The number of treatments varies according to the unique characteristics of your tumor. Treatment is usually administered five days a week for one to seven weeks.
Does the New York Proton Center offer clinical trials for lung and thoracic cancers?
With an active research program, we are pleased to offer a growing number of clinical trials for a variety of cancers. We are currently enrolling eligible patients for the following lung and thoracic study:
– NRG LU008 Phase III Prospective Randomized Trial of Primary Lung Tumor Stereotactic Body Radiation Therapy Followed by Concurrent Mediastinal Chemoradiation for Locally-Advanced Non-Small Cell Lung Cancer: NCT05624996
– MSK 22-098 Phase II Study of Hemithoracic Intensity-Modulated Pleural Radiation Therapy (IMPRINT) for Patients With Pleural Metastases From Thymic Malignancies: NCT05354570